Venous Thromboembolism (VTE) (e.g., deep vein thrombosis [DVT] and pulmonary embolism [PE]) are situations in which the the body inappropriately forms a clot, which if large enough, can impede the circulation of blood. In conventional medicine, the treatment for VTE is anticoagulant medication (i.e., Coumadin, Eliquis, Pradaxa, Xarelto). These medications will help to slowly dissolve the clot and prevent the occurrence of new clots. But why do these clots form in the first place?
Firstly, some people have inherited coagulation disorders that are genetic in nature. The most common of which is called Factor V Leiden. This article will focus on acquired, functional reasons that lead to hypercoagulability and VTE.
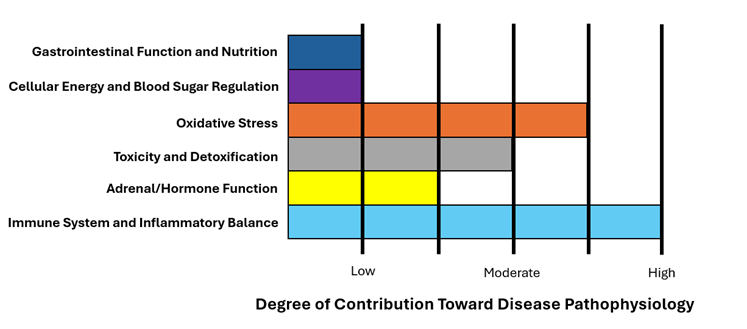
Inflammation/Immune System Dysfunction is a Major Component of the Pathophysiology of Blood Clot Formation
Remember that inflammation is a critical component of the body’s wound healing mechanism. Imagine you are preparing dinner and you accidentally cut yourself with a sharp knife. The laceration begins to bleed and the tissue starts to become inflamed. The body essentially uses inflammation to clot off the blood supply in that area to stop the bleeding. Mechanisms by which inflammation is able to accomplish this include increasing thrombin production, decreasing the activity of natural anticoagulant mechanisms, impairing fibrinolysis, and impairing vascular endothelial function. Acute inflammation in response to trauma or infection is a normal physiologic response.
However, if there is chronic, systemic inflammation then clots may form inappropriately within the venous system. Studies show that elevated C-Reactive Protein (CRP) levels (a marker of systemic inflammation) significantly increases the risk of VTE.1,2 Additionally, VTE risk is increased in both atopic conditions (i.e., asthma, allergies, eczema)3,4,5 and autoimmune conditions.6
VTE is a strong indicator that the system is severely inflamed and the immune system is hyperactive. We discussed in our article on autoimmunity that food sensitivities are an important cause of chronic inflammation and immune system dysfunction. Thus, a patient who has a VTE should strongly consider an elimination diet of potentially antigenic foods out of principle.
Oxidative Stress
Oxidative stress is also a key factor in the development of VTE. Reactive oxygen species affects the rigidity and pro-coagulant potential of red blood cells as well as damages endothelial cells and platelets, promoting thrombus formation. Oxidative stress is also highly connected to inflammation as discussed above. Research indicates that oxidative stress biomarkers (e.g., nitrated fibrinogen, MDA, ADMA) are significantly associated with an increased risk of VTE.7,8,9
Cancer is a pathological state that is well-known to cause a hypercoagulable state and increase the risk of VTE. This is not surprising given that cancer is closely connected to oxidative stress and inflammatory/immune system imbalance.
Hepatic Production of Clotting Factors and Methylation Dysfunction
Liver disease is well known to be associated with an increased risk of VTE.10 The liver produces various proteins that are involved in the clotting process. Fibrinogen, prothrombin, factors V, VII, VIII, IX, X, XI, and XII, and proteins C and S are all made by the liver and contribute to the clotting cascade process. If liver dysfunction leads to abnormal production of these clotting factors, this may also predispose to hypercoagulability. The association between liver disease and VTE is well recognized in cases of severe liver disease, such as cirrhosis; however, there is also a significantly increased risk of VTE in patients with Non-Alcoholic Fatty Liver Disease (NAFLD),11 a significantly more common problem.
Additionally, elevated levels of homocysteine (a marker of methylation dysfunction) is also associated with an increased risk of VTE.12,13 The degree of causality is controversial, however, as decreasing homocysteine levels with B-vitamin therapy has not been shown to reduce the incidence of VTE.14,15
Elevated Estrogen Is Related to Hypercoagulability
It is well known that exogenous, synthetic estrogen in the form of the oral contraceptive pill or synthetic hormone replacement therapy increases the risk of clotting and VTE. However, studies also show that elevated endogenous estrogen levels (i.e., estrogen dominance) is also related to increased risk.16 Additionally, elevated prolactin levels (which are generally secondary to chronically elevated estrogen) is also associated with increased risk of VTE.17 It is unclear whether or not the estrogen itself is causal or the underlying stress/inflammation that causes the elevated estrogen is the true causal factor.
Gastrointestinal Dysfunction and Cellular Energy/Mitochondrial Dysfunction May be Indirect Factors via Their Interaction with Other Imbalances
Gastrointestinal dysfunction is a major source of systemic inflammation. Recall that a large proportion of the immunologic/lymphatic tissue resides in and around the gastrointestinal system. Thus, GI irritation and dysbiosis can be an important physiologic factor in the development of systemic immune system imbalance. Additionally, mitochondrial dysfunction may lead to the backup of fuel and result in weight gain. Obesity is thought to be a risk factor for VTE, perhaps due to adipokines, which are inflammatory cytokines released by adipose (i.e., fat) tissue.
For those interested in learning more about these functional imbalances in more detail, consider reading the relevant sections in our manual.
References:
- Zacho J, Tybjaerg-Hansen A, Nordestgaard BG. C-reactive protein and risk of venous thromboembolism in the general population. Arterioscler Thromb Vasc Biol. 2010;30(8):1672-1678. doi:10.1161/ATVBAHA.109.198473
- Grimnes G, Isaksen T, Tichelaar YIGV, Brox J, Brækkan SK, Hansen JB. C-reactive protein and risk of venous thromboembolism: results from a population-based case-crossover study. Haematologica. 2018;103(7):1245-1250. doi:10.3324/haematol.2017.186957
- Majoor CJ, Kamphuisen PW, Zwinderman AH, et al. Risk of deep vein thrombosis and pulmonary embolism in asthma. Eur Respir J. 2013;42(3):655-661. doi:10.1183/09031936.00150312
- Lippi G, Favaloro EJ. Allergy and Venous Thromboembolism: A Casual or Causative Association. Semin Thromb Hemost. 2016;42(1):63-68. doi:10.1055/s-0035-1568876
- Wan J, Fuxench ZCC, Wang S, et al. Incidence of Cardiovascular Disease and Venous Thromboembolism in Patients With Atopic Dermatitis. J Allergy Clin Immunol Pract. 2023;11(10):3123-3132.e3. doi:10.1016/j.jaip.2023.08.007
- Zöller B, Li X, Sundquist J, Sundquist K. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2(3):171-183.
- Martinez M, Cuker A, Mills A, et al. Nitrated fibrinogen is a biomarker of oxidative stress in venous thromboembolism. Free Radic Biol Med. 2012;53(2):230-236. doi:10.1016/j.freeradbiomed.2012.05.004
- Reguig SB, Bouanane S, Merzouk H, Soufi N, Merzouk SA. Oxidative stress and thrombotic disorders: Study in patients with venous thromboembolism. Int J Health Sci Res. 2016;6:185-94.
- Chan JS, Hsiao PJ, Chiang WF, Roy-Chaudhury P. The Role of Oxidative Stress Markers in Predicting Acute Thrombotic Occlusion of Haemodialysis Vascular Access and Progressive Stenotic Dysfunction Demanding Angioplasty. Antioxidants (Basel). 2021;10(4):569. Published 2021 Apr 8. doi:10.3390/antiox10040569
- Søgaard KK, Horváth-Puhó E, Grønbaek H, Jepsen P, Vilstrup H, Sørensen HT. Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. Am J Gastroenterol. 2009;104(1):96-101. doi:10.1038/ajg.2008.34
- Di Minno MN, Tufano A, Rusolillo A, Di Minno G, Tarantino G. High prevalence of nonalcoholic fatty liver in patients with idiopathic venous thromboembolism. World J Gastroenterol. 2010;16(48):6119-6122. doi:10.3748/wjg.v16.i48.6119
- Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3(2):292-299. doi:10.1111/j.1538-7836.2005.01141.x
- Undas A, Brozek J, Szczeklik A. Homocysteine and thrombosis: from basic science to clinical evidence. Thromb Haemost. 2005;94(5):907-915. doi:10.1160/TH05-05-0313
- den Heijer M, Willems HP, Blom HJ, et al. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: A randomized, placebo-controlled, double-blind trial. Blood. 2007;109(1):139-144. doi:10.1182/blood-2006-04-014654
- Ray JG, Kearon C, Yi Q, Sheridan P, Lonn E; Heart Outcomes Prevention Evaluation 2 (HOPE-2) Investigators. Homocysteine-lowering therapy and risk for venous thromboembolism: a randomized trial. Ann Intern Med. 2007;146(11):761-767. doi:10.7326/0003-4819-146-11-200706050-00157
- Scheres LJJ, van Hylckama Vlieg A, Ballieux BEPB, et al. Endogenous sex hormones and risk of venous thromboembolism in young women. J Thromb Haemost. 2019;17(8):1297-1304. doi:10.1111/jth.14474
- van Zaane B, Squizzato A, Reuwer AQ, et al. Prolactin and venous thrombosis: indications for a novel risk factor?. Arterioscler Thromb Vasc Biol. 2011;31(3):672-677. doi:10.1161/ATVBAHA.110.209569