The Copper/Antioxidant System is the Battery
The engine of a car functions to burn gasoline into kinetic energy, however; in order to start the engine, it requires a battery. When you put your key in the ignition, it allows the battery to create an electrical “spark” to facilitate combustion. Without a functioning battery, the engine will not operate.
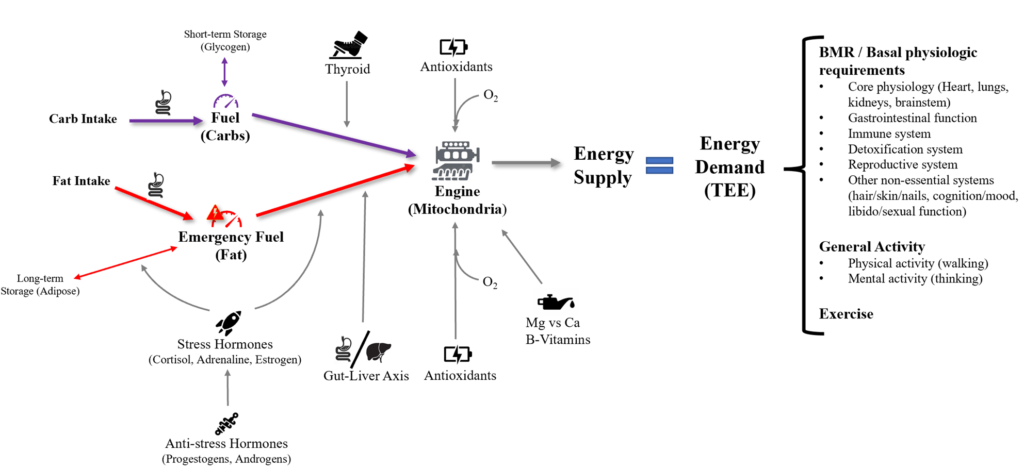
This is analogous to what is happening in the mitochondria (engine). The copper/antioxidant system maintains the proper redox environment and allows for proper enzymatic function for the electron transport chain. The electron transport chain essentially facilitates the electrical current down an energy gradient as the final step in mitochondrial ATP synthesis. Oxygen serves as the final electron acceptor of this series, generating water as a byproduct. The term “oxygen activation” refers to this process of oxygen receiving the electron.
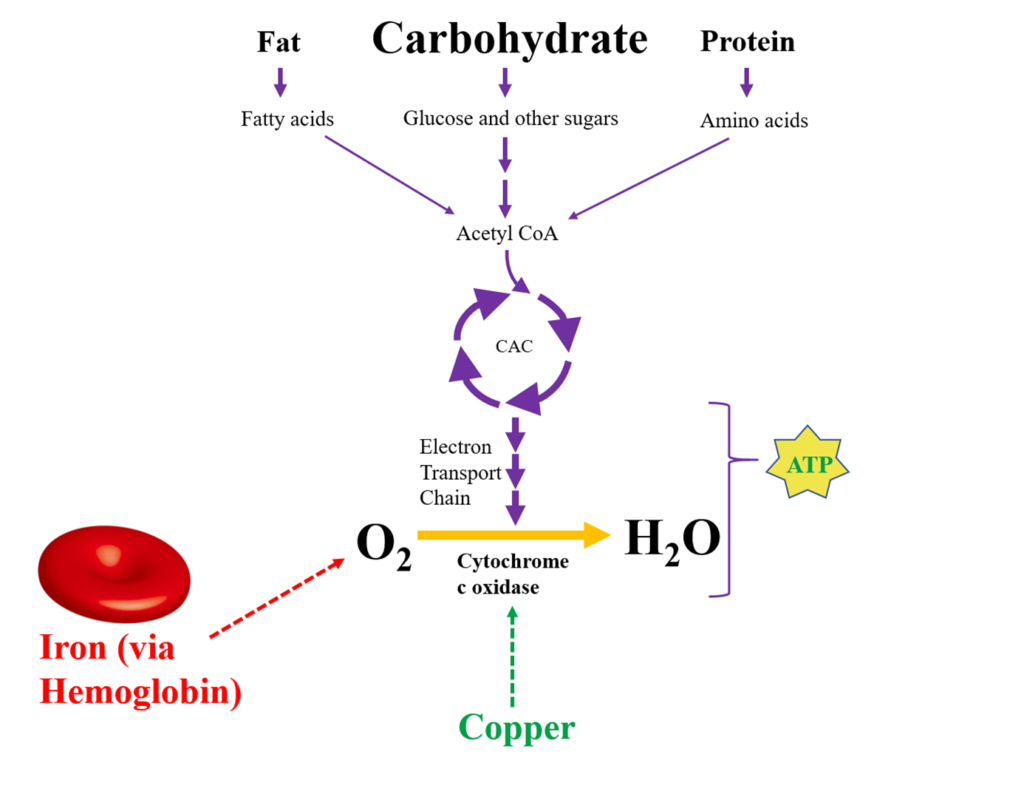
Failure to Activate Oxygen Efficiently Results in Oxidative Stress (Rust)
If the oxygen activation system is dysfunctional, then oxygen and iron will not be utilized properly. Oxygen will be shunted into a pathway that leads to the formation of toxic superoxide molecules (O2–) and the accumulation of an assortment of Reactive Oxygen Species (ROS) including superoxides, peroxides, and hydroxyl radicals. Additionally, oxygen will combine with iron to generate iron oxide, which is essentially a form of rust. Iron oxides can form deposits and promote ongoing oxidation in the liver, intestines, bone marrow, joints, and brain. Organic tissues that encounter ROS become damaged. For example, a tomato that sits on the counter for too long will become oxidized, turn brown, and wither away. This parallels what may take place in our bodies when oxidative stress overwhelms the antioxidant system. Maintenance of the copper/antioxidant system and minimization of ROS is of critical importance to the overall metabolic function and longevity of the organism.
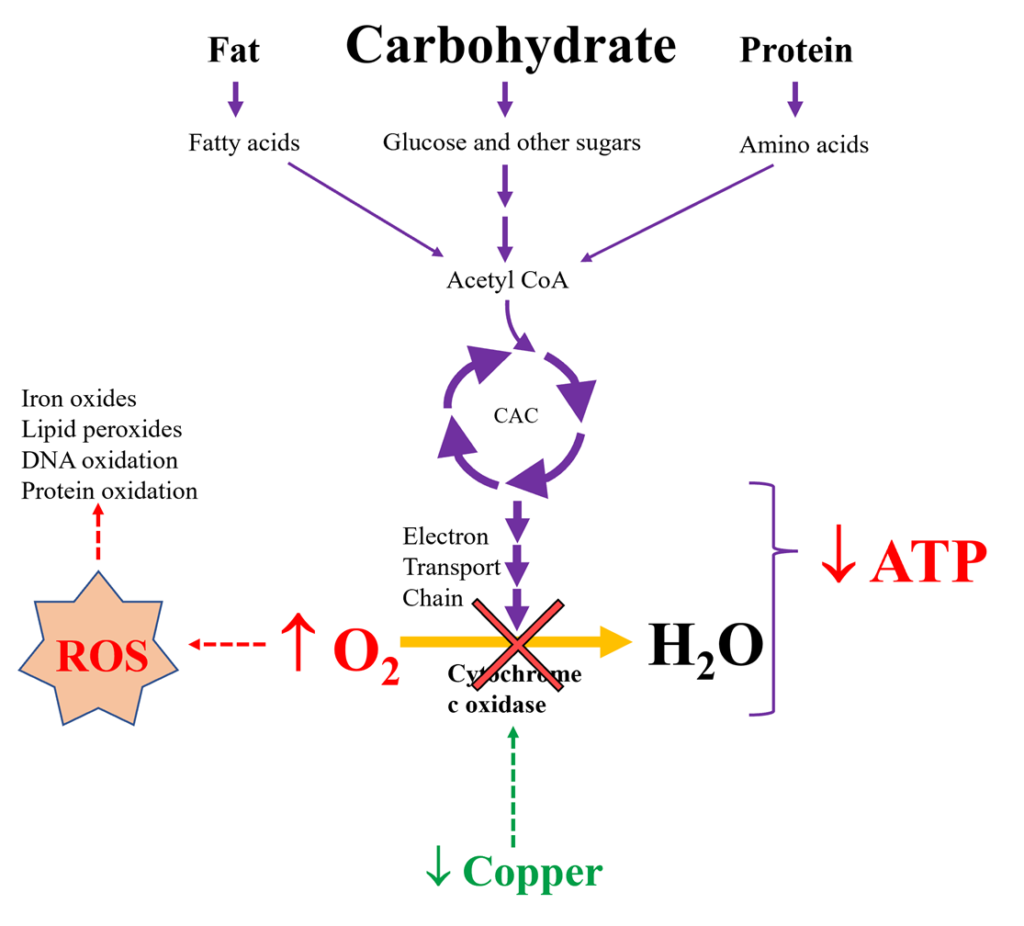
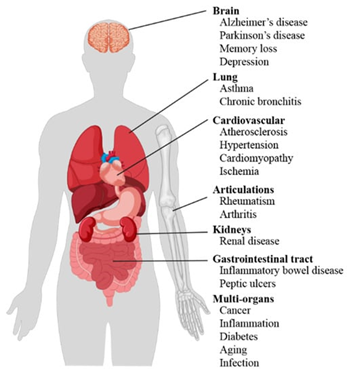
Oxidative Stress is Directly Involved in the Pathophysiology of Many Chronic Diseases
Higher levels of oxidative stress are associated with a greater risk of all-cause mortality, as well as with the development of cardiovascular disease, cancer, and a host of other disease states (see below). The idea that accumulation of oxidative stress is a major contributor to the aging process is sometimes referred to as the free radical theory of aging.
How we Evaluate Oxidative Stress
Antioxidant Markers:
- Serum Ceruloplasmin – bioavailable copper level
- Serum Glutathione – level of the glutathione, the body’s master antioxidant
- Vitamin A (Retinol) – level of another important fat-soluble antioxidant
Oxidant Markers:
- Urine F2-Isoprostane/Creatinine Ratio – a byproduct of omega-6 PUFA oxidation, considered by many to be the “gold standard” for measuring oxidative stress levels
- Serum Hemoglobin and Ferritin – elevated levels suggest iron overload

